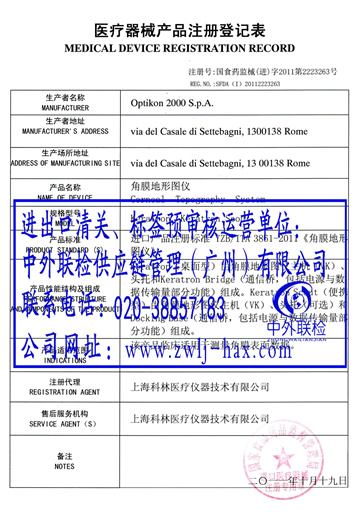
According to the requirements of the relevant documents of the National FDA, all imported medical devices need to apply to CFDA for registration and obtain the registration certificate of medical devices. Considering that many foreign and domestic customers are not familiar with the collation and other requirements of domestic documents related to the registration certificate, in order to meet the requirements of customers and better reflect the supply chain services, China-Foreign Joint Inspection will organize industry elites in Beijing in 2011. By 2016, the office has successfully applied for the registration certificate of medical devices for more than 300 enterprises.